Class 12 Chemistry CBSE chemical Kinetics Board Questions
Here we provide Class 12 chemistry important notes,board questions and predicted questions with Answers for chapter Chemical Kinetics. These important notes,board questions and predicted questions are based on CBSE board curriculum and correspond to the most recent Class 12 chemistry syllabus. By practising these Class 12 materials, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 12 Board examinations as well as other entrance exams such as NEET and JEE.
2020
Q1
The rate constant for the first order decomposition of N2O2 is given by the
following equation:
k = (2.5 x 1014 s-1)e(-25000K)/T
Calculate Ea for this reaction and rate constant if it's half life period be 300 minutes.
solutions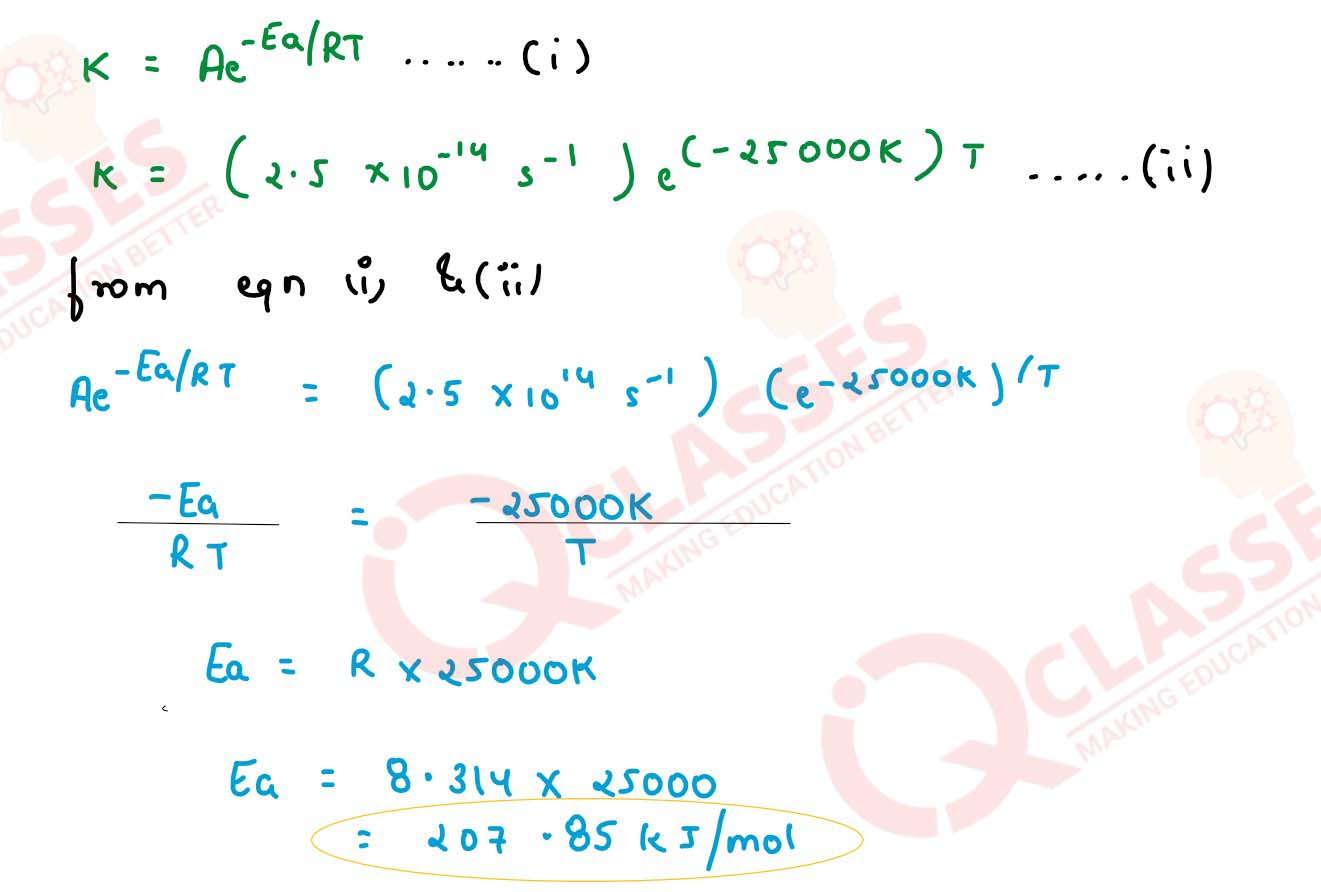
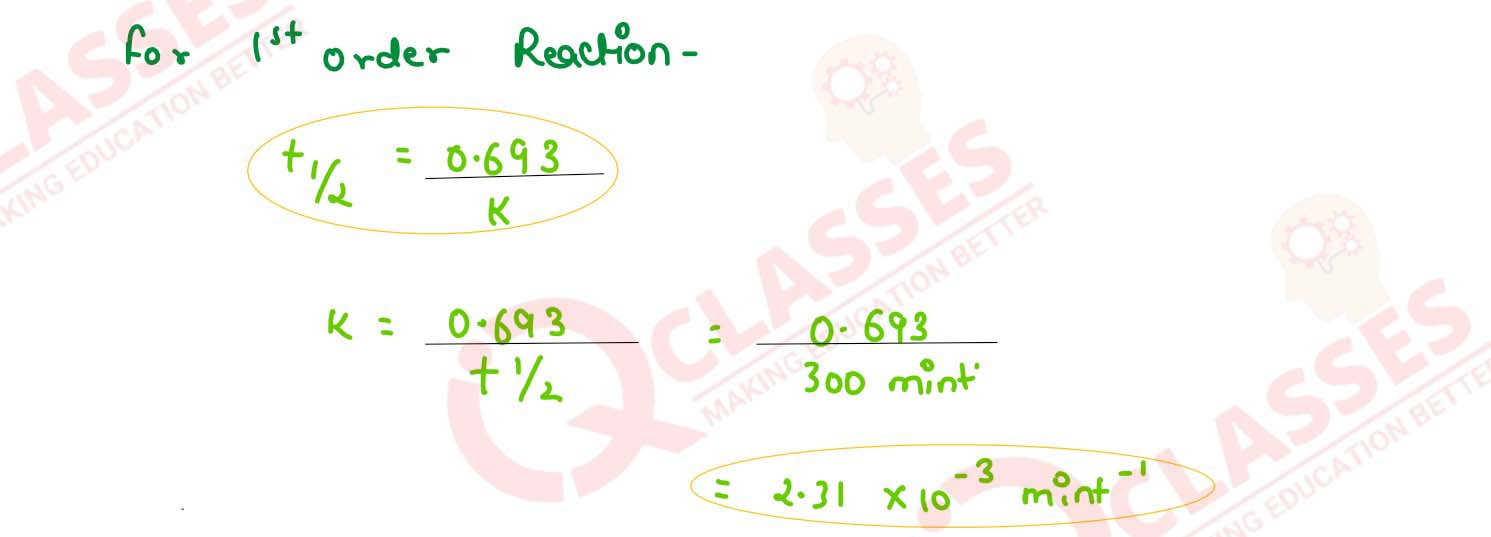
k = (2.5 x 1014 s-1)e(-25000K)/T
Calculate Ea for this reaction and rate constant if it's half life period be 300 minutes.
solutions


2019
Q2
The decomposition of NH3 on platinum surface is zero order reaction. If the rate constant
K is 4 x 10-3 Ms-1, how long will it take to reduce the initial concentration
of ammonia from 0.1 M to 0.064 M.
solutions

solutions


2018
Q3
For the reaction :
2 N2O5(g) → 4NO2(g) + O2(g)
The rate of formation of NO2(g) is 2.8 x 10 -3 Ms-1 . Calculate the rate of disappearance of N2O5(g)
solutions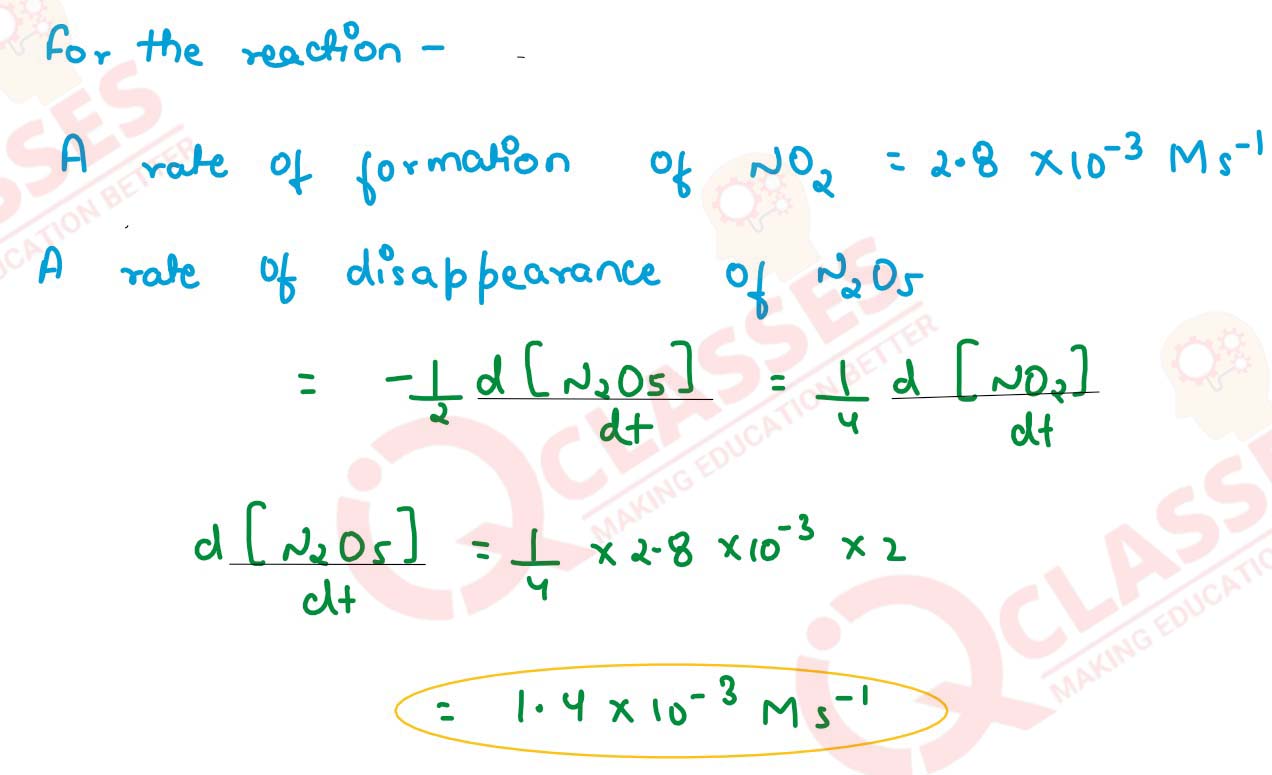
2 N2O5(g) → 4NO2(g) + O2(g)
The rate of formation of NO2(g) is 2.8 x 10 -3 Ms-1 . Calculate the rate of disappearance of N2O5(g)
solutions

2017
Q4
A first order reaction takes 20 minutes for 25% decomposition.Calculate the time when 75% of the
reaction will be completed.
(Given : log 2 =0.03010 , log 3 = 0.4771, log 4 = 0.6021)
solutions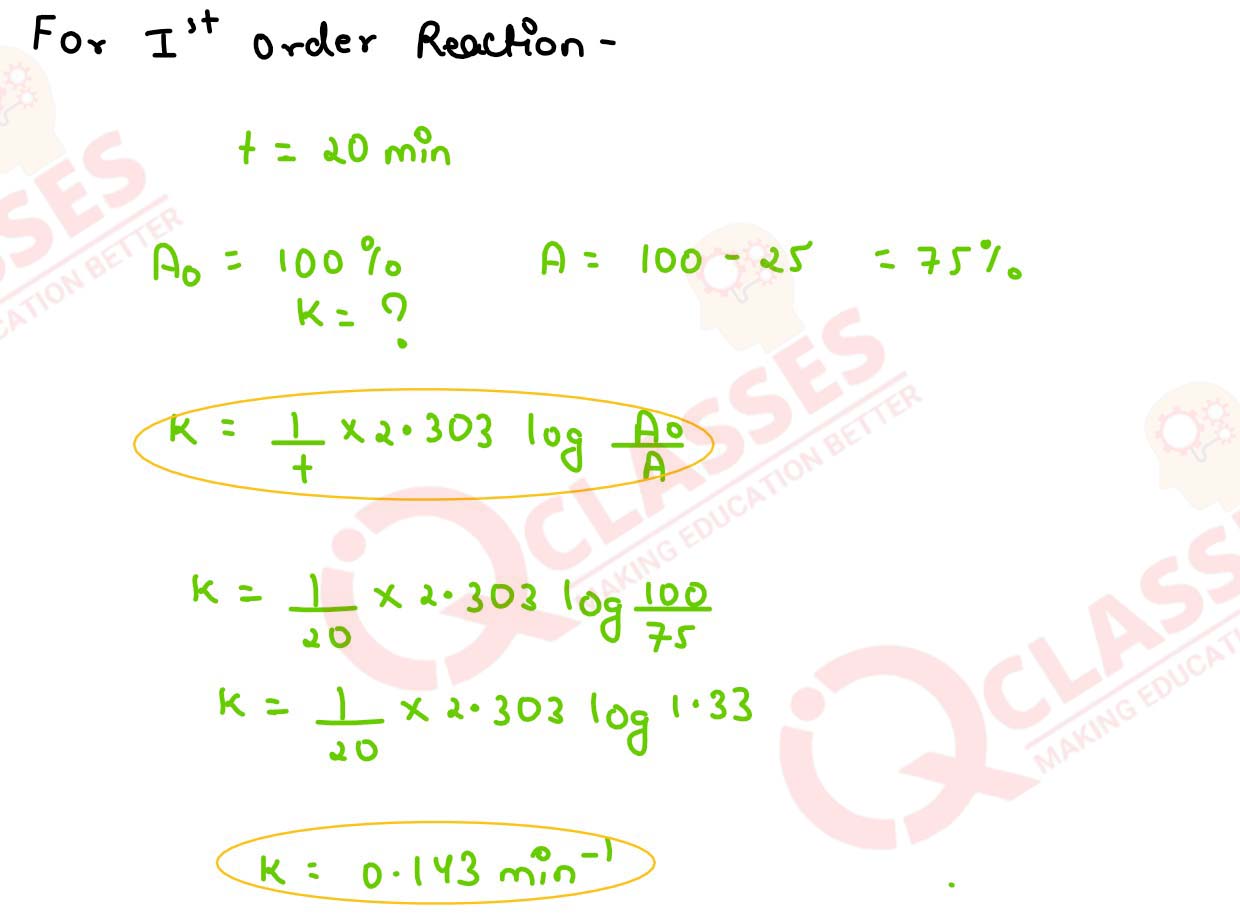

(Given : log 2 =0.03010 , log 3 = 0.4771, log 4 = 0.6021)
solutions


2016
Q5
For the first order thermal decomposition reaction,the following data were obtained
C2H5 Cl(g) → C2H4(g) + HCl(g)
Calculate the rate constant
(Given : log 2 =0.03010 , log 3 = 0.4771, log 4 = 0.6021)
solutions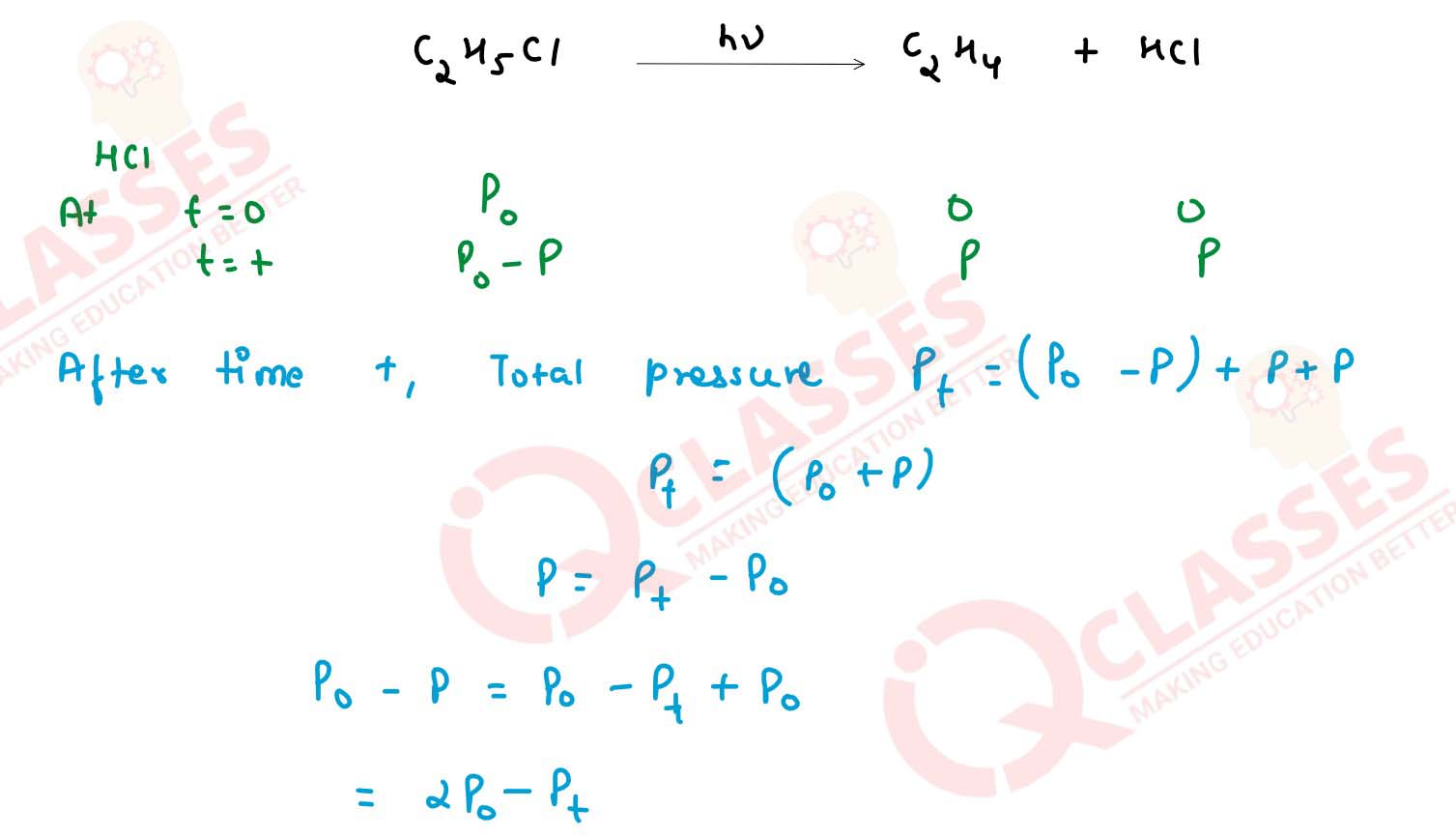
C2H5 Cl(g) → C2H4(g) + HCl(g)
| Time/sec | Total Pressure/atm |
| 0 | 0.30 |
| 300 | 0.50 |
(Given : log 2 =0.03010 , log 3 = 0.4771, log 4 = 0.6021)
solutions


Reach Us
SERVICES
- ACADEMIC
- ON-LINE PREPARATION
- FOUNDATION & CRASH COURSES
CONTACT
B-54, Krishna Bhawan, Parag Narain Road,
Near Butler Palace Colony Lucknow
Contact:+918081967119

Add a comment