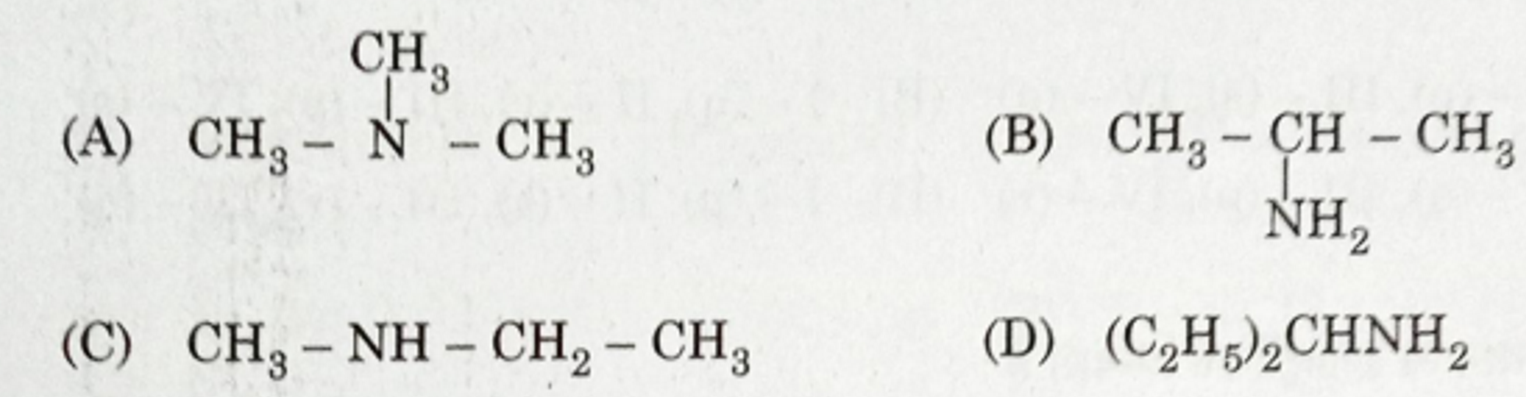Class 12 CBSE Chemistry Question Paper 2024
Maximum Marks: 70
Time Allowed: Three hours
All questions are compulsory.
The question paper has five sections and 33 questions. All questions are compulsory.
Section–A has 16 questions of 1 mark each; Section–B has 5 questions of 2 marks each;
Section– C has 7 questions of 3 marks each; Section– D has 2 case-based questions of 4
marks each; and Section–E has 3 questions of 5 marks each.
There is no overall choice. However, internal choices have been provided in some
questions. A student has to attempt only one of the alternatives in such questions.
Wherever necessary, neat and properly labeled diagrams should be drawn
Section-A
Question 1
Question No. to 16 are Multiple Choice type questions carrying 1 mark each
The molar conductivities of Ca2+ and Cl- are 119.0 and 76.3 S cm2mol-1 respectively. The value of limiting molar conductivity of CaCl2 will be: (a) 195.3 Scm2mol-1 (b) 43.3 Scm2mol-1 (c) 314.3 Scm2mol-1 (d) 271.6 Scm2mol-1
Question 2
Consider the following reaction :
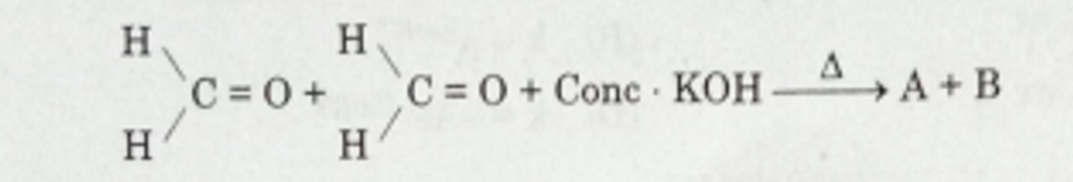
Identify А апd В from the given options :
(A) А — Methanol, В — potassium formate
(В) А — Ethanol, В — Potassium formate
(С) A-Methanol,B-Ethanol
(D) А — Methanol, В — Potassium aeetate
Question 3
Which of the following acids represents Vitamin C ?
(A) Saccharic acid
(C) Ascorbic acid
(B) Gluconic acid
(D) Benzoic acid
Question 4
Rosenmund reduction is used for the preparation of Aldehydes. The catalyst used in this reaction is
(A) Pd-BaSO4
(B) Anhydrous AlCl3
(C) Iron (III) oxide
(D) HgSO4
Question 5
Which alkyl halide from the given options will undergo SNl reaction faster ?
(A) (CH3)3C-Br
(B) (CH3)2CH-Br
(C) CH3-CH2-Br
(D) (CH3)3C-CH2-Br
Question 6
From the elements of 3d series given below, which element shows the maximum number of oxidation states ?
(A) Scandium
(B) Manganese
(C) Chromium
(D) Titanium
Question 7
The correct Mathematical expression Of Arrhenius equation is:
(A) k = —A eEa/RT
(B) k eEa/RT
(C) k = Ae-Ea/RT
(D) k = —Ae-Ea/RT
Question 8
Question 9
Nucleophilic addition of Grignard reagent to ketones followed by hydrolysis with dilute acids forms :
(A) Alkene
(B) Primary alcohol
(C) Tertiary alcohol
(D) Secondary alcohol
Question 10
In a given graph of zero order reaction, the slope and intercept are :
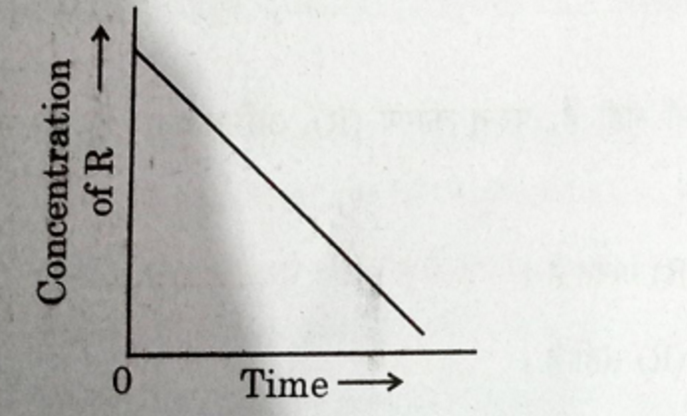
(A) Slope = k, Intercept = [R]o
(B) Slope = -k, Intercept = [R]o
(C) Slope = k/2.303, Intercept =In[R]o
(D) Slope = -k/2.303, Intercept = In A
Question 11
Match the reagents required for the given reactions :
I. Oxidation of primary alcohols to aldehydes
II. Butan-2-one to Butan-2-ol
III. Bromination of Phenol to 2, 4, 6- Tribromophenol IV. Dehydration of propan-2-ol to propene to (s) Bromine water propene
(A) I-(r),II-(p),III-(s),IV-(q)
(B) I-(q),II-(r),III-(p),IV-(s)
(C) I-(s),II-(q),III-(p),IV-(r)
(D) I-(p),II-(s),III-(r),IV-(q)
Question 12
The general electronic configuration of d-block elements is :
(A) (n — 1) d1-10ns1-2
(B) (n — 1) d10ns1-2
(C) (n — 1) d10ns2-3
(D) (n — 1) d0ns1-2
For questions number 13 to 16, two statements are given — one labelled as
Assertion (A) and the other labelled as Reason (R). Select the correct
answer to these questions from the codes (A), (B), (C) and (D) as given
below :
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the
correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the
correct explanation of the Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
Question 13
Assertion (A) : p-nitrophenol is less acidic than phenol.
Reason (R) : Nitro group is electron withdrawing and helps in the stabilisation of p-nitrophenoxide ion.
Question 14
Assertion (A) : Benzoic acid does not undergo Friedel — Crafts reaction.
Reason (R) : Carboxyl group is deactivating and the catalyst aluminium chloride gets bonded to the carboxyl group.
Question 15
Assertion (A) : Fructose is a reducing sugar.
Reason (R) : Fructose does not reduce Fehling solution and Tollen's reagent.
Question 16
Assertion (A) : For a Daniell cell, Zn/Zn2+(1M) || Cu2+ (1M)/Cu with Eocell = 1.1 V, if the external opposing potential is more than 1.1 V, the electrons flow from Cu to Zn.
Reason (R) : Cell acts like a galvanic cell.
Question 17
Question 18
18 g of a non-volatile solute is dissolved in 200 g of H2O freezes at 272.07 K. Calculate the molecular mass of solute (Kf for water = 1.86 K kg mol-1)
Question 19
(a) Which compound in the given pair would undergo SN2 reaction at a faster rate and why ?
CH3-CH2-I and CH3-CH2-Br
(b) Arrange the following compounds in the increasing order of their boiling points :
Butane, 1—Bromobutane, I—Iodobutane, I—Chlorobutane
Question 20
(a) Write the stepwise mechanism of nucleophilic addition reactions in the carbonyl compounds.
OR
(b) How will you convert the following :
(i) Toluene to benzoic acid.
(ii) Ethanol to 3- Hydroxybutanal
Question 21
(a) What happens when glucose reacts with bromine water ? Write chemical equation.
(b) Two bases are mentioned below, identify which is present in DNA and which one is present in RNA :
(i) Thymine, (ii) Uracil.
Section-C
Question 22
(a) Draw the geometrical isomers of the given complex :
[Pt(en)2Cl2]2+
(b) Write the electronic configuration for d4 ion if ▵o < P on the basis of crystal field theory.
(c) What is meant by a unidentate ligand ? Give an example.
Question 23
Question 24
Write chemical equations for the following reactions : (Do any three)
(a) Hydroboration — oxidation reaction
(b) Williamson Synthesis
(c) Friedel-Crafts Alkylation of Anisole
(d) Reimer-Tiemann Reacåon
Question 25
(a) Give chemical tests to distinguish between the following pairs of compounds :
(i) Phenol and Benzoic acid
(ii) Propanal and Propanone
(b) Which one of the given compounds is a stronger acid and why ?
CH2>FCH2>CH2>COOH or CH3CHFCH2COOH
Question 26
Explain the following terms :
(a) Essential amino acids
(b) Peptide bond
(c) Denaturation
Question 27
(a) Write the IUPAC name of the given compound
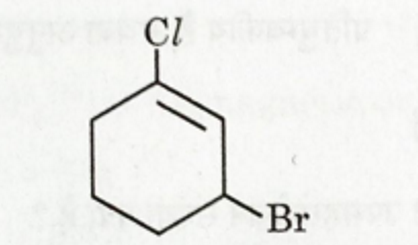
(b) The presence of -NO2 group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution reactions.Give reason to explain the above statement
(c) What happens when ethyl chloride is treated with alcoholic potassium hydroxide
Question 28
Show that the time required for 99.9% completion in a first order reaction is 10 times of half-life (t1/2) of the reaction [log 2 = 0.3010, log 10 = 1].
Question 29
The following questions are case-based questions. Read the case carefully and answer the questions that follow
The nature of bonding structure of the coordination compound can be explained to some extent by valence bond theory, The central metal atom/ion makes available a number of vacant orbital equal to its coordination number. The appropriate atomic orbital (s, p and d) of the metal hybridise to give a set of equivalent orbitals of definite geometry such as square planar,tetrahedral, octahedral and so on. A strong covalent bond is formed only when the orbitals overlap to the maximum extent. The d-orbitals involved in the hybridisation may be either inner d-orbitals i.e (n-1)d or outer d-orbitals i.e. nd. The complexes formed are caned inner orbital complex (low spin complex) and outer orbital complex (high spin complex) respectively. Further, the complexes can be paramagnetic or diamagnetic in nature. The drawbacks Of this theory are that this involves number of assumptions and also does not explain the colour of the complex.
Answer the following questions:
(a) Predict whether [CoF6]3- is diamagnetic or paramagnetic and why?
[Atomic number: Co=27]
(b) What is the coordination number of Co in [Co(en)2Cl2]+
(c) (i) Write the IUPAC name of the given complex:
[Pt(NH3)2Cl2]2+
(ii) Explain [Co(NH3)6]3+ is an inner orbital or outer orbital complex
OR
Using valence bond theory,deduce the shape and hybridisation of [Ni(NH3)6]2+[Atomic number of Ni=28]
Question 30
In a galvanic cell,chemical energy of a redox reaction is converted into electrical energy,whereas in an electrolytic cell the redox reaction occurs on passing electricity.The simplest galvanic cell is in Zn rod is placed in a solution of ZnSO4 and Cu rod is placed in a solution of CuSO4.The two rods are connected by a metallic wire through a voltmeter.The two solutions are joined by a salt bridge.The difference between the two electrode potentials of the two electrodes is known as electromotive force.In the process of electrolysis,the decomposition of a substance takes place by passing an electic current.One mole of electric charge when passed through a cell will discharge half a mole of a divalent metal ion such as Cu2+.This was first formulated by Faraday in the form of laws of electrolysis
Answer the following questions:
(a) What is the function of a salt bridge in a galvanic cell?
(b) When does galvanic cell behave like an electrolytic cell?
(c) Can copper sulphate solution be stored in a pot made of zinc? Explain with the help of the value of Eo cell
(EoCu2+/Cu=0.34 V)
(EoZn2+/Zn=-0.76 V)
OR
(c) How much charge in terms of faraday is required for the following:
(i) 1 mol of MnO4- to Mn2+
(ii) 1 mol of H2O to O2
Section-E
Question 31
Attempt any five of the following :
(a) Why Zinc is not regarded as a transition element ?
(b) What is Lanthanoid contraction ?
(c) Why is first ionization enthalpy of chromium lower than that of Zn ?
(d) Why are transition elements good catalysts?
(e) Compounds of transition metals are generally coloured. Give reason.
(f) Out of KMnO4 and K2MnO4,which one is paramagnetic and why?
(g) Complete the following ionic equation:
Cr2O72-+14H++6e--->
Question 32
(a) (i) Define reverse osmosis
(ii) Why are aquatic species more comfortable in cold in comparison to warm water?
(iii) A solution containing 2g of glucose (M=180 gmol-1) in 100g of water is prepared at 303K.If the vapour pressure of pure water at 303K is 32.8 mm Hg.What would be the vapour pressure of the solution?
(b) (i) (1)What is Hinsberg's reagent?
(2) Arrange the following compounds in the increasing order of their basic strength in gaseous phase:
C2H5.NH2.(C2H5)2.NH
(ii) Give reasons for the following:
(i) Methyl amine is more basic than aniline.
(ii) Aniline readily reacts with bromine water to give 2,4,6-tribromoaniline
(iii) Primary amines have higher boiling points than tertiary amines
OR
(b) (i) Predict whether Van't Hoff factor will be less or greater than one,when ethanoic acid is dissolved in benzene
(ii) Define ideal solution
(iii) Calculate the mass of CaCl2(molar mass=111 gmol-1) to the dissolved in 500g of water to lower its freezing point by 2K,assuming that CaCl2 undergoes complete dissociation
(Kf for water=1.86 K kg mol-1)
(Kf for water=1.86 K kg mol-1)